DAT Fundamentals: Biology & Chemistry Essentials
Master foundational concepts in biology, general chemistry, organic chemistry, and perceptual ability for DAT success.
DAT Fundamentals: Biology & Chemistry Essentials
Prepare for the Dental Admission Test with free flashcards covering the four critical sections of this challenging exam. This lesson introduces you to biology (the highest-yield section with 40 questions), general chemistry, organic chemistry, and perceptual ability—all essential for achieving a competitive DAT score. You'll learn strategic approaches to tackle the 90-minute science section efficiently while building a strong foundation in the most tested concepts.
Welcome to DAT Preparation! 🦷
The DAT is a comprehensive exam that assesses your readiness for dental school across multiple disciplines. Unlike many standardized tests, the DAT requires you to rapidly switch between biology, chemistry, and spatial reasoning—all within tight time constraints. This lesson focuses on high-yield concepts that appear most frequently on the exam, along with proven test-taking strategies to maximize your score.
What Makes the DAT Unique:
- 🧬 Biology dominance: 40 out of 100 science questions
- ⏱️ Time pressure: 90 minutes for all science sections combined
- 🧪 Breadth over depth: Tests wide knowledge rather than advanced theory
- 🔺 Perceptual Ability: Unique spatial reasoning section not found on most exams
💡 Pro Tip: The DAT rewards breadth of knowledge over deep specialization. Focus on understanding core concepts across all topics rather than mastering any single area.
Core Concepts: Biology (Highest Yield!) 🧬
Biology comprises 40% of your science score, making it the most important section to master. Focus on these high-yield areas:
Cell Biology & Molecular Biology
Cell Membrane Structure - The foundation of cellular function:
- Phospholipid bilayer: Hydrophilic heads face outward, hydrophobic tails face inward
- Fluid mosaic model: Proteins float within the membrane like icebergs in a sea
- Selective permeability: Controls what enters and exits the cell
Key Transport Mechanisms:
| Transport Type | Energy Required? | Direction | Example |
|---|---|---|---|
| Simple Diffusion | No (passive) | High → Low concentration | O₂, CO₂ across membrane |
| Facilitated Diffusion | No (passive) | High → Low concentration | Glucose through protein channels |
| Active Transport | Yes (ATP) | Low → High concentration | Na⁺/K⁺ pump |
| Osmosis | No (passive) | Water moves to high solute | Water entering cells |
🧠 Mnemonic for Active Transport: "Active needs ATP" - if it's moving against the gradient, it needs energy!
Organelles and Their Functions:
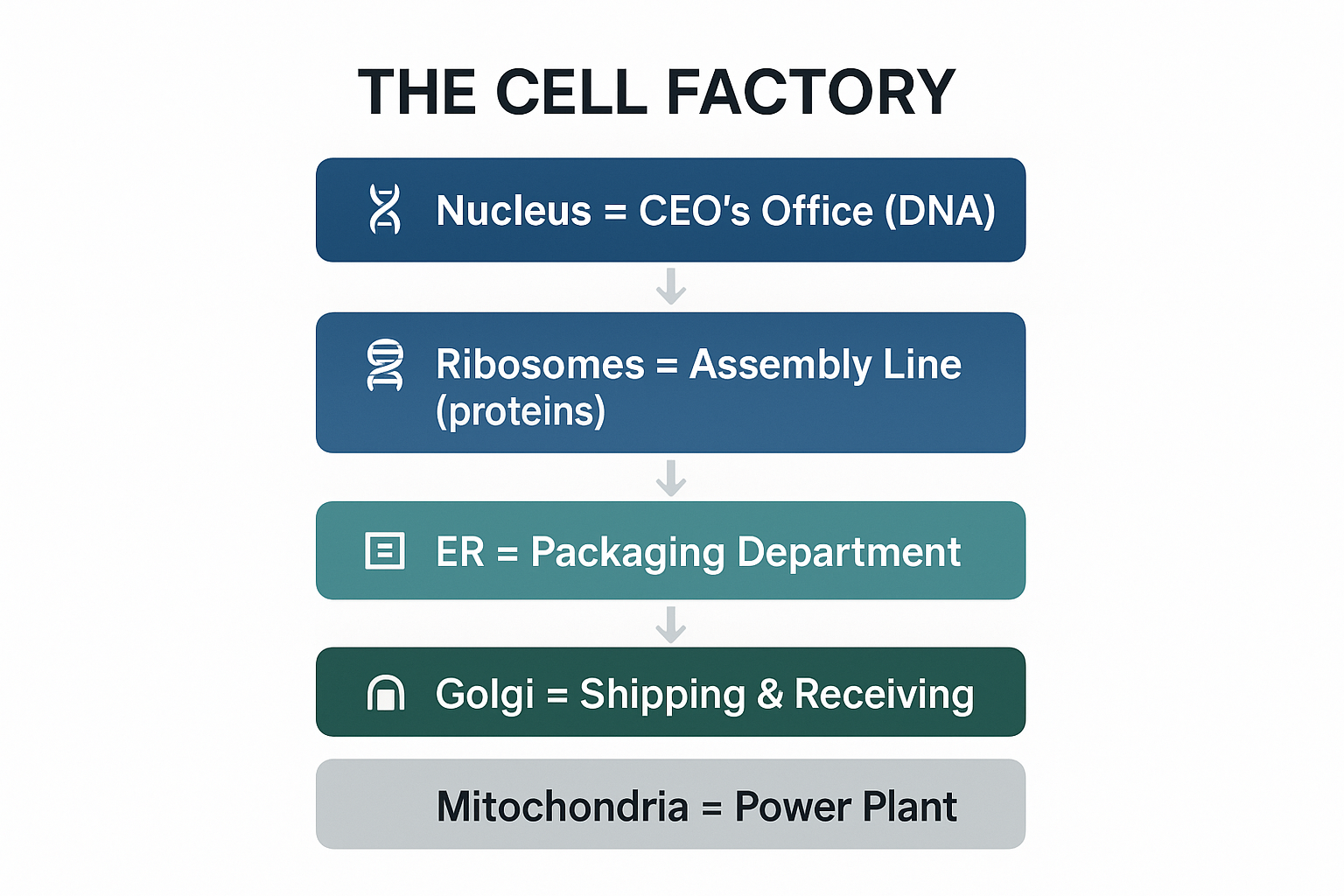
View original ASCII
THE CELL FACTORY ┌─────────────────────────────────────────┐ │ 🧬 Nucleus = CEO's Office (DNA) │ │ │ │ │ ↓ │ │ 📜 Ribosomes = Assembly Line (proteins)│ │ │ │ │ ↓ │ │ 📦 ER = Packaging Department │ │ │ │ │ ↓ │ │ 🏤 Golgi = Shipping & Receiving │ │ │ │ │ ↓ │ │ ⚡ Mitochondria = Power Plant (ATP) │ └─────────────────────────────────────────┘
Genetics & DNA
DNA Replication - "Semi-conservative" means each new DNA molecule contains one old strand and one new strand:
- Helicase unzips the double helix (think: helicopter blades spinning)
- Primase lays down RNA primers (starting points)
- DNA polymerase adds nucleotides in the 5' → 3' direction
- Ligase seals gaps between Okazaki fragments on the lagging strand
🧠 Mnemonic for DNA Replication Enzymes: "Help Please Dear Lucy"
- Helicase, Primase, DNA polymerase, Ligase
Protein Synthesis Flow:
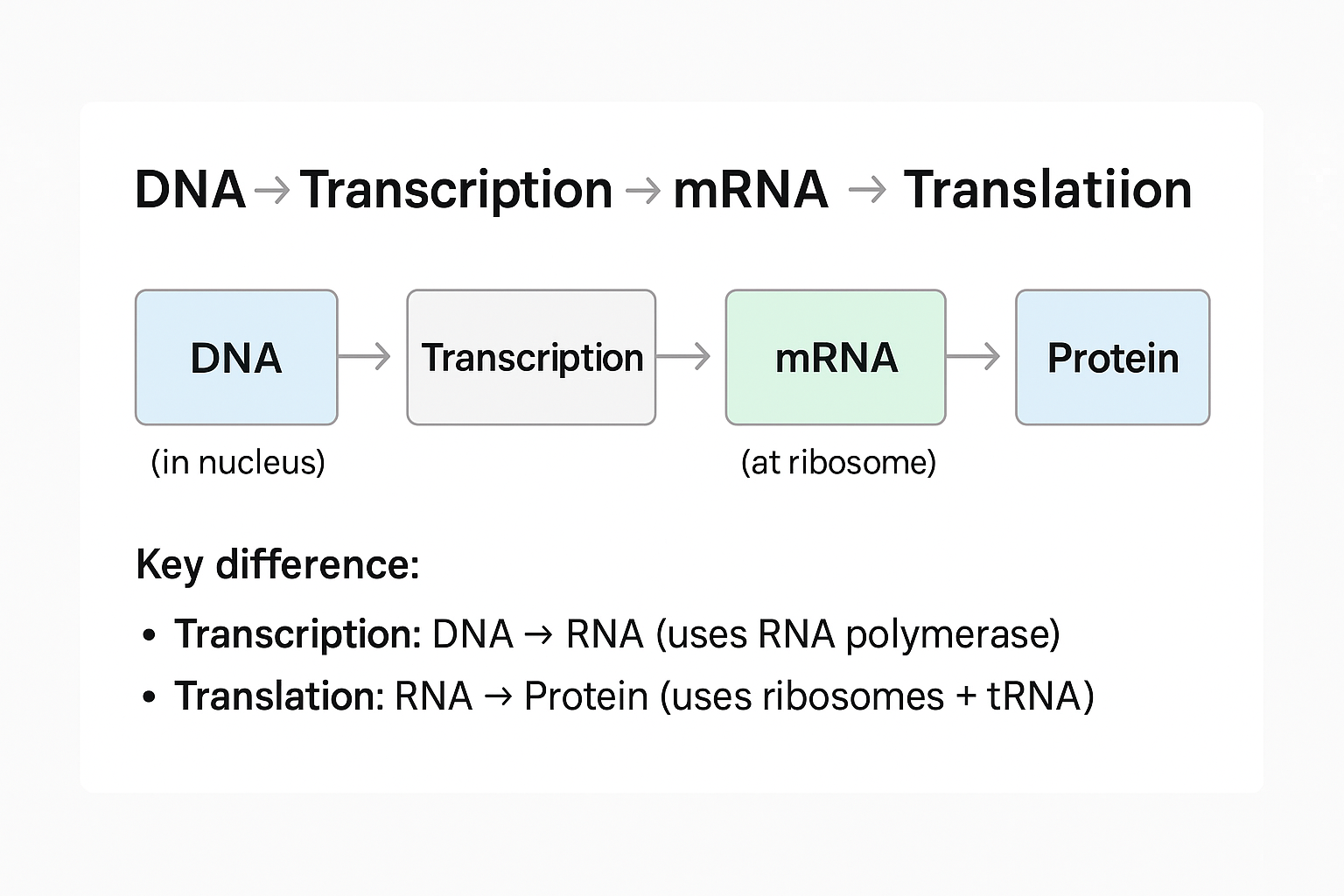
View original ASCII
DNA → Transcription → mRNA → Translation → Protein 🧬 (in nucleus) 📜 (at ribosome) 🔧Key difference: • Transcription: DNA → RNA (uses RNA polymerase) • Translation: RNA → Protein (uses ribosomes + tRNA)
Codon Chart Essentials:
- Start codon: AUG (codes for methionine)
- Stop codons: UAA, UAG, UGA
- 🧠 Mnemonic for Stop Codons: "U Are Away, U Are Gone, U Go Away"
Evolution & Ecology
Natural Selection - Darwin's four key principles:
📋 Natural Selection Checklist
| 1. Variation | Individuals in a population differ |
| 2. Inheritance | Traits are passed to offspring |
| 3. Overproduction | More offspring than can survive |
| 4. Differential Survival | Best-adapted individuals survive and reproduce |
Population Ecology Terms:
- Carrying capacity (K): Maximum population size an environment can sustain
- Limiting factors: Resources that restrict population growth (food, water, space)
- Exponential growth: J-shaped curve (unlimited resources)
- Logistic growth: S-shaped curve (limited resources, levels off at K)
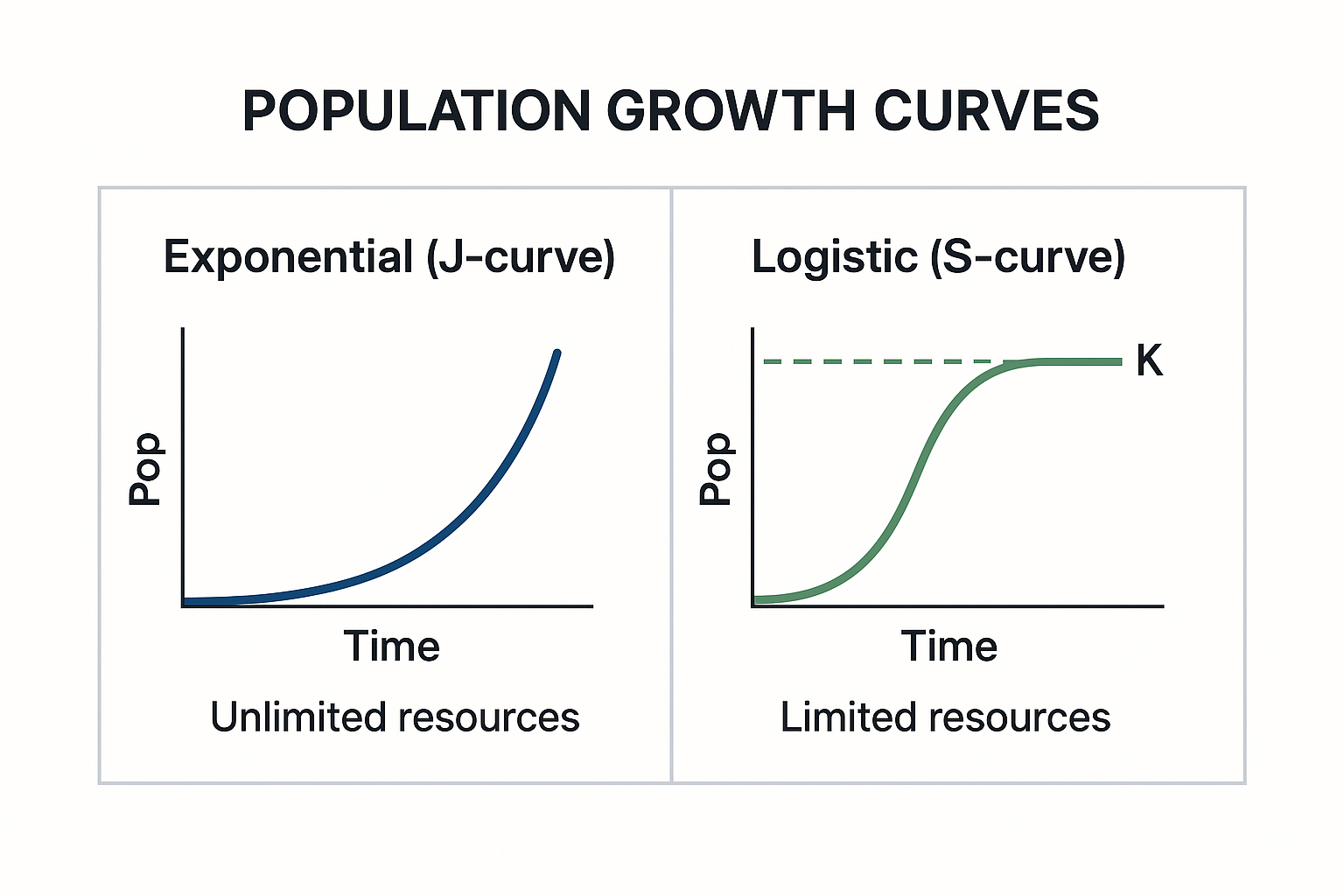
View original ASCII
POPULATION GROWTH CURVESExponential (J-curve) Logistic (S-curve) │ │ K │ ╭ │ ╱ Pop │ ╱ │ │ │ ╱ │ ╱ │ ╱ │╱ └─────── Time └────── Time Unlimited resources Limited resources
Core Concepts: General Chemistry 🧪
Atomic Structure & Periodic Trends
Periodic Table Trends - Master these for quick problem-solving:
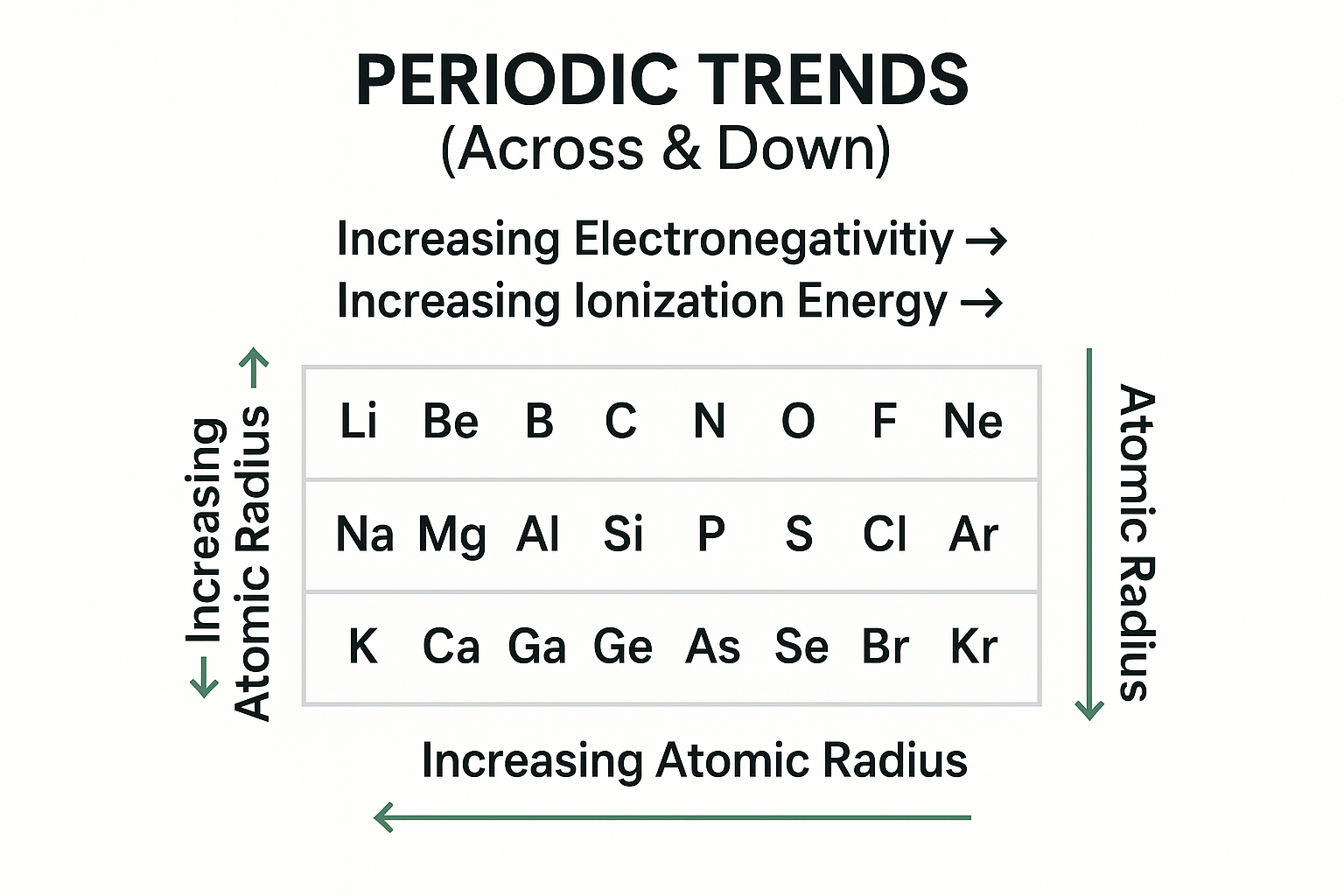
View original ASCII
PERIODIC TRENDS (Across & Down)→ Increasing Electronegativity → → Increasing Ionization Energy → ← Increasing Atomic Radius ←┌──────────────────────────────────────┐ │ Li Be B C N O F Ne│ ↑ │ │ │ │ Na Mg Al Si P S Cl Ar │ │ Increasing │ │ │ Atomic │ K Ca Ga Ge As Se Br Kr │ │ Radius └──────────────────────────────────────┘ ↓
💡 Test Strategy: Questions often ask you to compare element properties. Remember: Francium is the largest atom, Fluorine is the most electronegative (opposite ends!).
Quantum Numbers - Describe electron location:
| Symbol | Name | Describes | Values |
|---|---|---|---|
| n | Principal | Energy level/shell | 1, 2, 3, 4... |
| l | Angular momentum | Subshell shape | 0 to (n-1) 0=s, 1=p, 2=d, 3=f |
| mₗ | Magnetic | Orbital orientation | -l to +l |
| mₛ | Spin | Electron spin | +½ or -½ |
Stoichiometry & Chemical Reactions
Mole Conversions - The heart of stoichiometry:
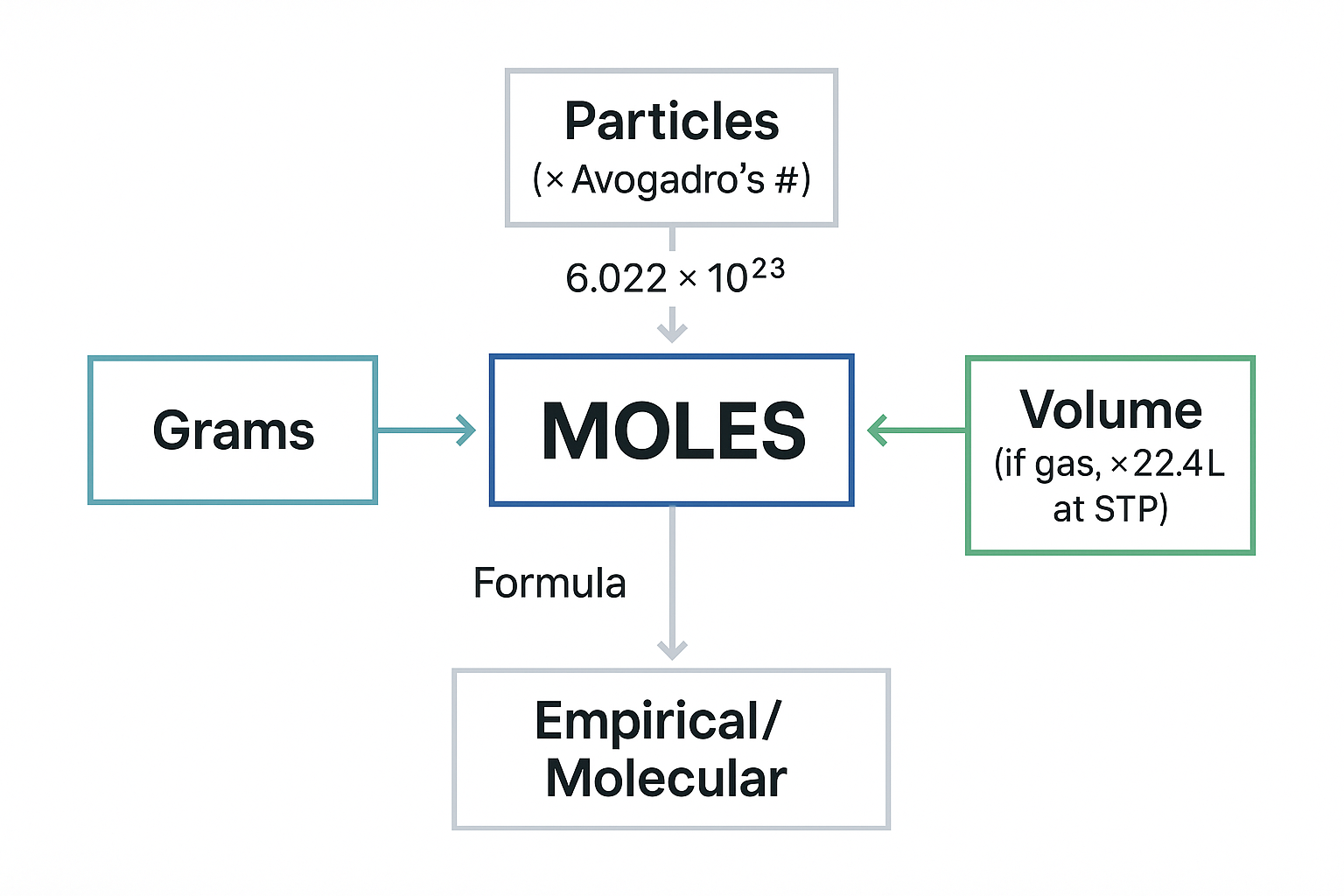
View original ASCII
┌─→ Particles (× Avogadro's #) ←┐
│ 6.022 × 10²³ │
│ │
Grams ←→ MOLES ←→ Volume (if gas, × 22.4L at STP)
│ ↕ │
│ Formula │
└─→ Empirical/Molecular ←────────┘
🧠 Mnemonic for Mole Conversions: "Mary Goes Picking Violets" = Moles, Grams, Particles, Volume
Balancing Equations Strategy:
- Balance metals first
- Balance non-metals second
- Balance hydrogen third
- Balance oxygen last
- Check your work!
Acids, Bases & pH
pH Scale Essentials:
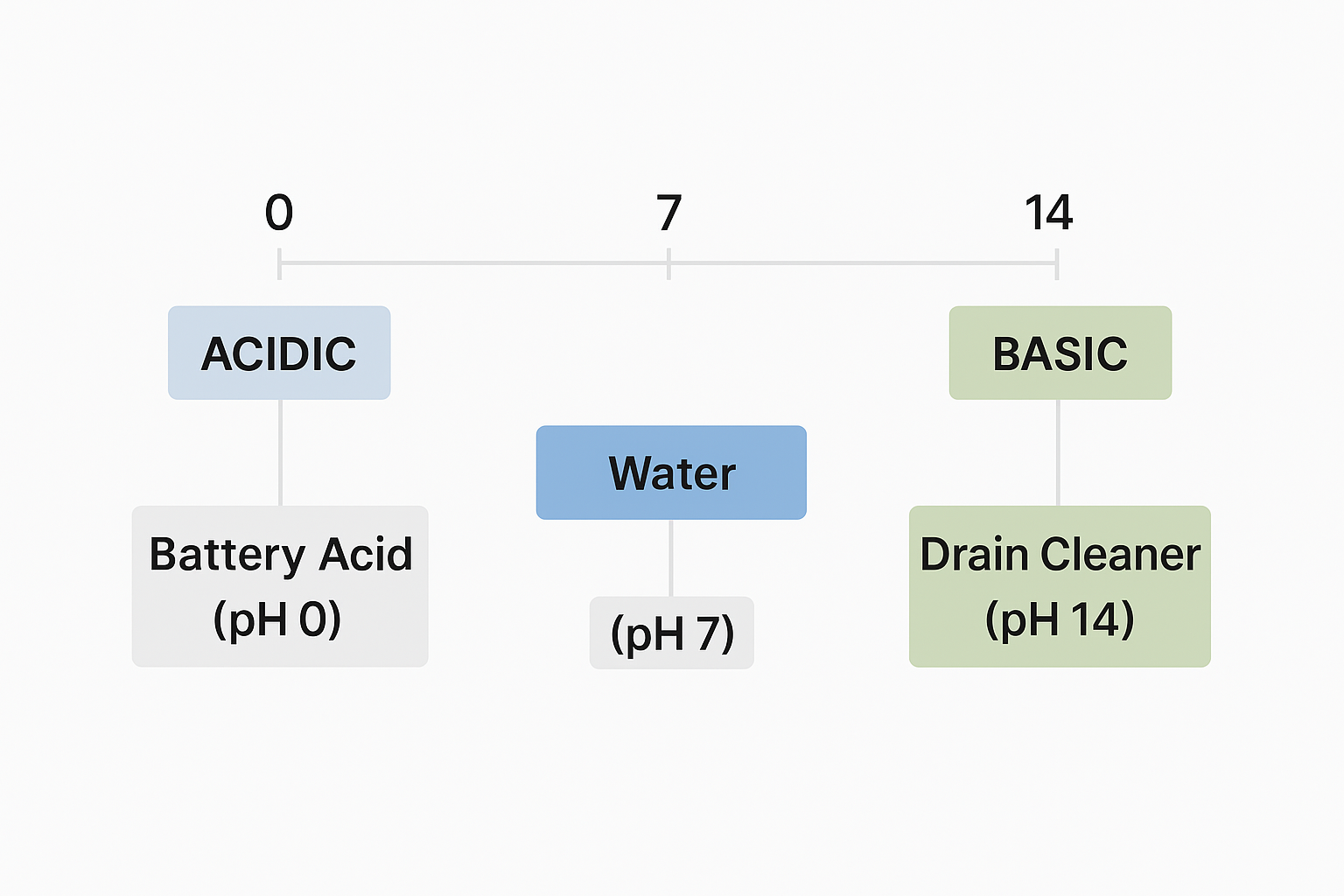
View original ASCII
0 ─────────── 7 ─────────── 14 │ ACIDIC │ BASIC │ │ │ │ Battery Acid Water Drain Cleaner (pH 0) (pH 7) (pH 14)
Key Formulas:
- pH = -log[H⁺]
- pOH = -log[OH⁻]
- pH + pOH = 14
- [H⁺][OH⁻] = 1.0 × 10⁻¹⁴ (at 25°C)
Strong Acids (memorize these - they dissociate 100%):
- HCl (hydrochloric), HBr (hydrobromic), HI (hydroiodic)
- HNO₃ (nitric), H₂SO₄ (sulfuric), HClO₄ (perchloric)
🧠 Mnemonic: "High Clerk Brought In No Stupid Papers" (HCl, HBr, HI, HNO₃, H₂SO₄, HClO₄)
Core Concepts: Organic Chemistry 🧪
Functional Groups (Must Memorize!)
| Functional Group | Structure | Suffix/Prefix | Example |
|---|---|---|---|
| Alkane | C-C single bonds | -ane | Methane (CH₄) |
| Alkene | C=C double bond | -ene | Ethene (C₂H₄) |
| Alkyne | C≡C triple bond | -yne | Ethyne (C₂H₂) |
| Alcohol | -OH | -ol | Ethanol (C₂H₅OH) |
| Aldehyde | -CHO | -al | Methanal (HCHO) |
| Ketone | C=O (middle) | -one | Propanone (acetone) |
| Carboxylic Acid | -COOH | -oic acid | Ethanoic acid (acetic) |
| Ester | -COO- | -oate | Methyl ethanoate |
| Amine | -NH₂ | -amine | Methylamine |
🧠 Priority Order for Naming (highest to lowest): Carboxylic Acid > Ester > Aldehyde > Ketone > Alcohol > Amine > Alkene > Alkyne > Alkane
Stereochemistry Basics
Chirality - A molecule is chiral if it has a carbon with 4 different groups (asymmetric carbon):
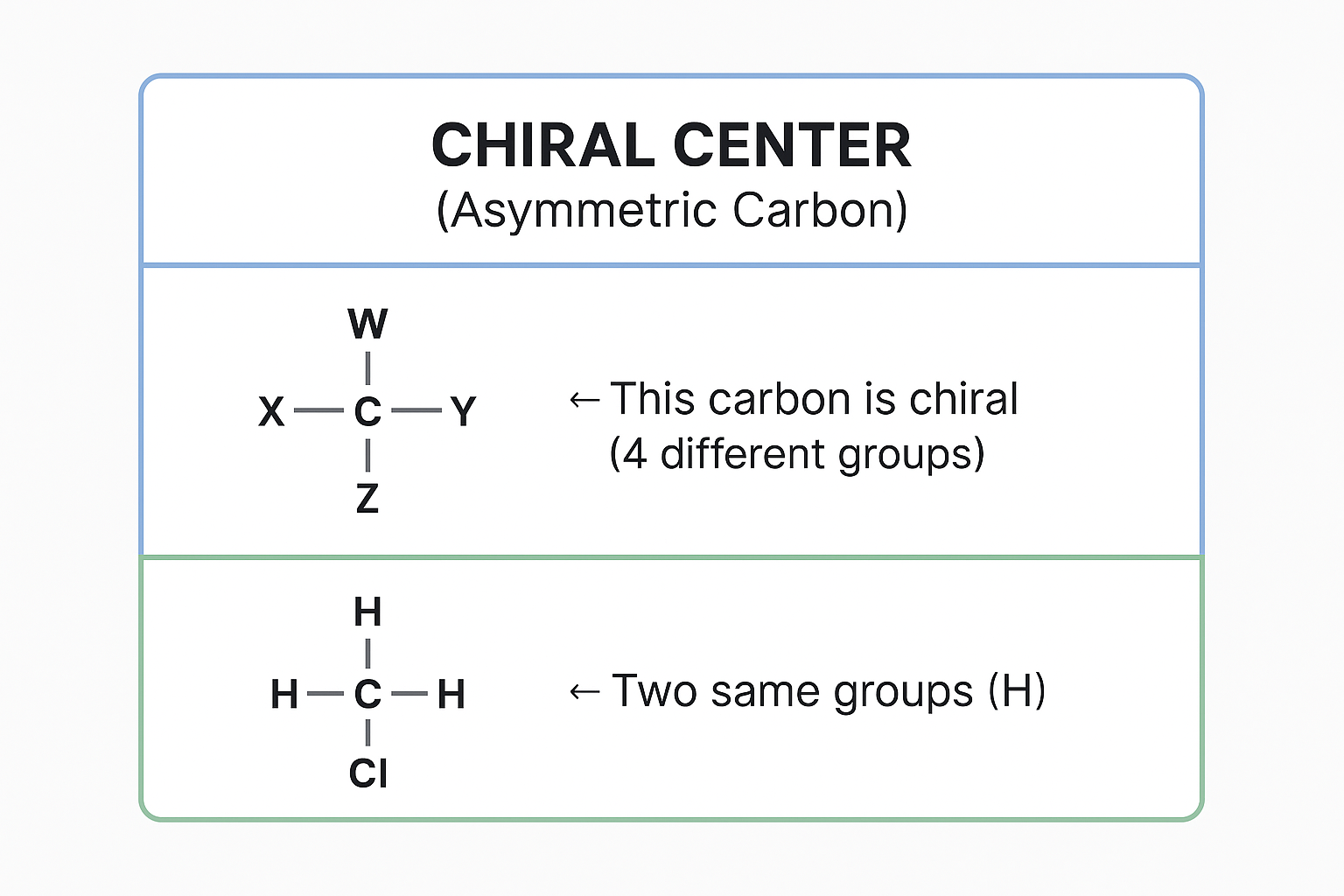
View original ASCII
CHIRAL CENTER (Asymmetric Carbon)W │ X───C───Y ← This carbon is chiral │ (4 different groups) ZNON-CHIRAL EXAMPLES: H │ H───C───H ← Two same groups (H) │ Cl
R/S Configuration - Use Cahn-Ingold-Prelog priority rules:
- Rank substituents by atomic number (higher = higher priority)
- Orient molecule so lowest priority points away
- Draw arrow from 1→2→3
- Clockwise = R ("rectus"), Counterclockwise = S ("sinister")
💡 DAT Tip: You won't need to assign complex R/S configurations on the DAT, but you must recognize chiral centers and understand enantiomers vs. diastereomers.
Key Reaction Mechanisms
Nucleophile vs. Electrophile:
- Nucleophile ("nucleus-loving"): Electron-rich, negative or neutral, attacks positive sites
- Examples: OH⁻, NH₃, H₂O, RO⁻
- Electrophile ("electron-loving"): Electron-deficient, positive or neutral, accepts electrons
- Examples: H⁺, carbocations (R⁺), carbonyl carbon (C=O)
🧠 Memory Aid: "Nucleophile has numerous electrons" vs. "Electrophile is empty of electrons"
Core Concepts: Perceptual Ability Test (PAT) 🔺
The PAT section is unique to the DAT and tests your spatial reasoning—critical for dental procedures. Practice is essential!
Angle Ranking 📐
Strategy: Estimate angles by comparing to reference angles:
- 90° = Right angle (square corner)
- 45° = Half of 90° (diagonal of square)
- 180° = Straight line
- 60° = Angle in equilateral triangle
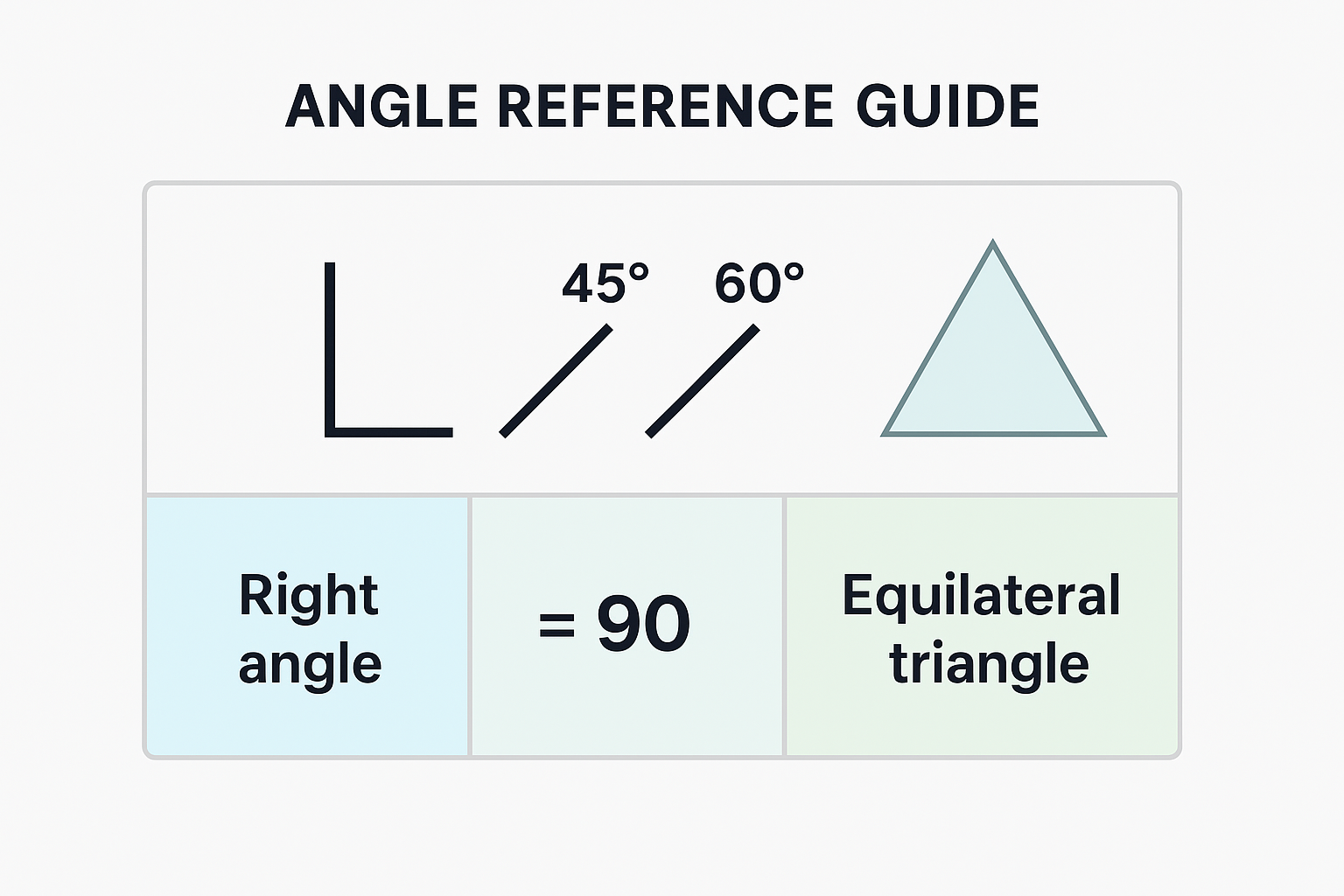
View original ASCII
ANGLE REFERENCE GUIDE│ ╱ ╱╲ │ ╱45° ╱60°╲────┼──── = 90° Equilateral triangle │ Right angle
💡 Quick Tip: Angles less than 45° are "acute and cute" (small). Angles between 45-90° are still acute. 90-180° are obtuse ("obtuse = obese = big").
Hole Punching 🕳️
Strategy: Visualize the fold sequence carefully:
- Track each fold direction (horizontal, vertical, diagonal)
- When hole is punched, it goes through ALL layers
- Unfold in REVERSE order
- Each layer adds symmetrical holes
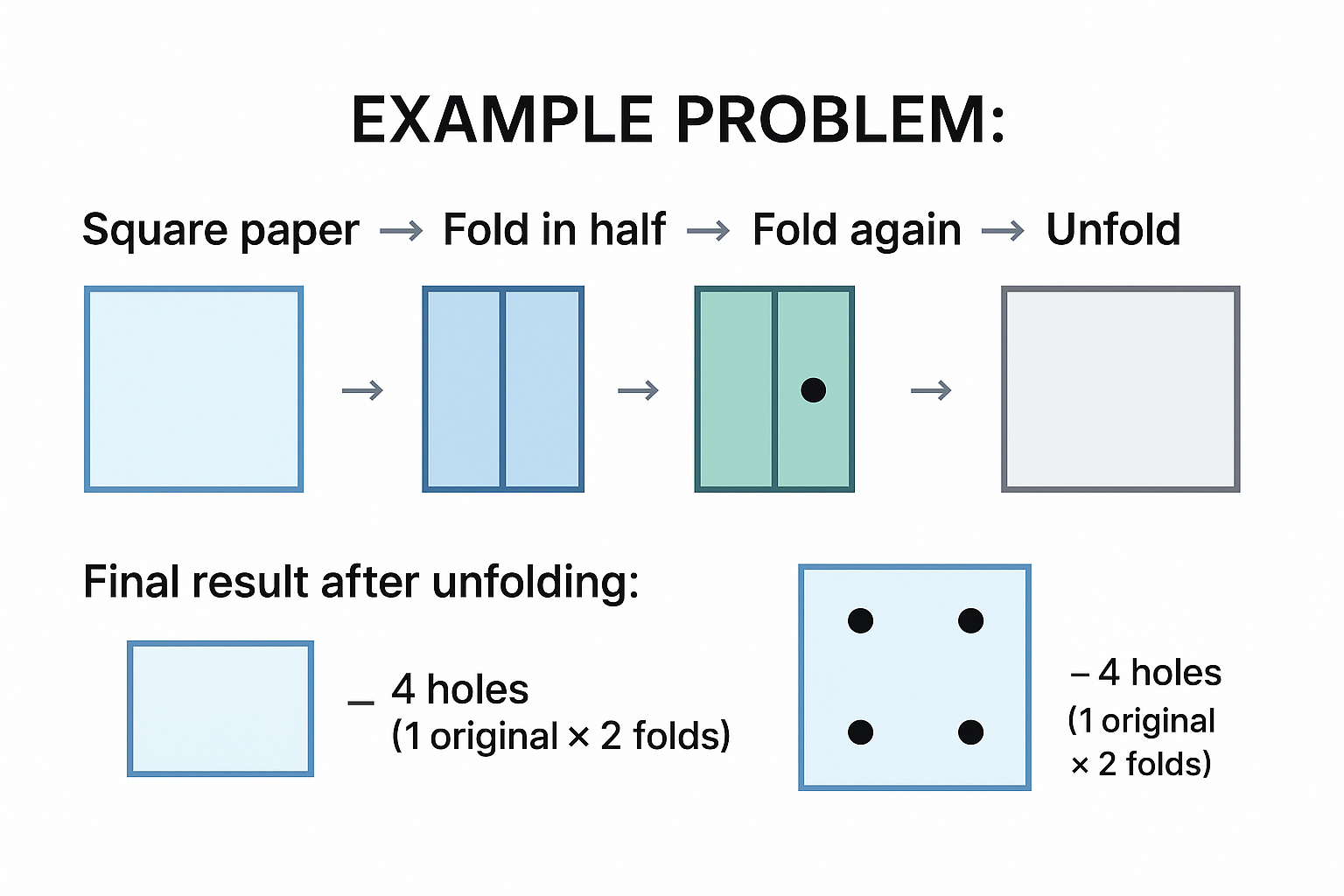
View original ASCII
EXAMPLE PROBLEM:Square paper → Fold in half → Fold again → Punch hole → Unfold ┌────┐ ┌──┐ ┌┐ ┌┐ │ │ → │ │ → ││ → ●│ └────┘ └──┘ └┘ └┘
Final result after unfolding: ┌────┐ │● ●│ ← 4 holes (1 original × 2 folds) │● ●│ └────┘
Cube Counting 📦
Strategy: Systematically count cubes by category:
- Fully hidden cubes (no faces visible)
- One face showing
- Two faces showing (edge cubes)
- Three faces showing (corner cubes)
💡 Hidden Cube Formula: For a rectangular stack, count internal cubes = (length-2) × (width-2) × (height-2)
Pattern Folding 📋
Strategy: Look for "impossible" configurations:
- ❌ Adjacent sides in the flat pattern can't be opposite in 3D
- ❌ Check shading/patterns align correctly at edges
- ✅ Identify the base first, then build up mentally
View Recognition 👁️
Top-Front-End Views: Practice visualizing objects from different angles:

View original ASCII
3D OBJECT VIEWSTOP VIEW FRONT VIEW END VIEW(looking down) (from front) (from side)
┌─────┐ ┌──┐ ┌───┐ │ │ │ │ │ │ │ ■ │ └──┘ │ │ └─────┘ └───┘</pre>
💡 Strategy: Eliminate wrong answers by finding ONE feature that doesn't match.
Test-Taking Strategies for the 90-Minute Science Section ⏱️
Time Management Blueprint:
| Section | Questions | Recommended Time | Per Question |
|---|---|---|---|
| Biology | 40 | 35-40 minutes | ~60 seconds |
| General Chemistry | 30 | 20-25 minutes | ~50 seconds |
| Organic Chemistry | 30 | 20-25 minutes | ~50 seconds |
| Review/Check | — | 5-10 minutes | — |
The "Flag and Move" System 🚩
Three-Pass Strategy:
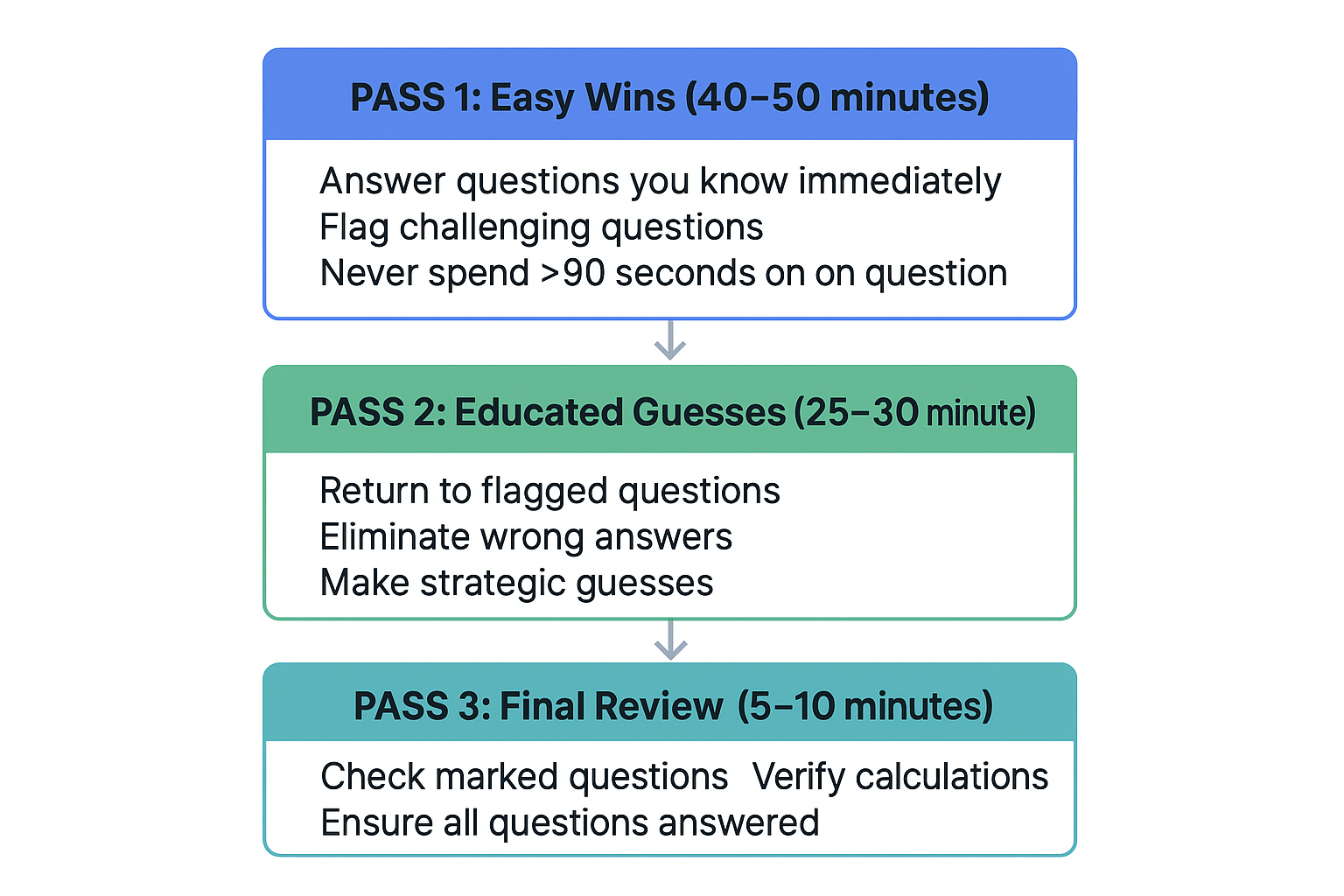
View original ASCII
PASS 1: Easy Wins (40-50 minutes) ↓ Answer questions you know immediately Flag challenging questions Never spend >90 seconds on one questionPASS 2: Educated Guesses (25-30 minutes) ↓ Return to flagged questions Eliminate wrong answers Make strategic guesses
PASS 3: Final Review (5-10 minutes) ↓ Check marked questions Verify calculations Ensure all questions answered
Process of Elimination (POE) Techniques ✂️
For Biology Questions:
- ❌ Eliminate extreme answers ("always," "never," "only")
- ❌ Remove options with unfamiliar terminology (often distractors)
- ✅ Choose answers that reflect mainstream biology concepts
For Chemistry Calculations:
- ❌ Eliminate answers with wrong units
- ❌ Remove values that are orders of magnitude off
- ✅ Estimate before calculating (prevents calculation errors)
For Organic Chemistry:
- ❌ Eliminate reactions that violate basic principles (charge balance, octet rule)
- ❌ Remove products with wrong functional groups
- ✅ Focus on electron flow (nucleophile attacks electrophile)
🧠 Golden Rule: When stuck between two answers, choose the one that represents a MORE FUNDAMENTAL concept. The DAT tests core principles, not obscure exceptions.
Examples with Detailed Explanations
Example 1: Biology - Cell Transport 🧬
Question Type: A patient receives an IV solution that is hypotonic relative to their blood cells. What will happen to the red blood cells?
Solution Process:
| Step | Analysis | Reasoning |
|---|---|---|
| 1 | Define "hypotonic" | Lower solute concentration outside cell than inside |
| 2 | Determine water movement | Water moves FROM hypotonic solution INTO cell (osmosis) |
| 3 | Predict outcome | Cell swells as water enters, may burst (lyse) |
Answer: The red blood cells will swell and potentially undergo lysis (burst).
💡 DAT Application: This concept appears in questions about IV solutions, kidney function, and plant cell turgor pressure. Remember: water follows solute ("water goes where the salt is").
Example 2: General Chemistry - pH Calculation 🧪
Question Type: What is the pH of a 0.01 M HCl solution?
Solution Process:
| Step | Work | Result |
|---|---|---|
| 1 | Recognize HCl is a strong acid | 100% dissociation |
| 2 | [H⁺] = 0.01 M = 1 × 10⁻² | Molarity of H⁺ |
| 3 | pH = -log[H⁺] = -log(10⁻²) | Apply pH formula |
| 4 | pH = 2 | Final answer |
Answer: pH = 2
💡 Mental Math Shortcut: For strong acids/bases in "1 × 10⁻ⁿ" format, pH = n (for acids) or pOH = n (for bases). Example: 10⁻³ M HCl → pH = 3 immediately!
Example 3: Organic Chemistry - Functional Group Priority 🧪
Question Type: Name this compound: CH₃-CH₂-CH(OH)-CHO
Solution Process:
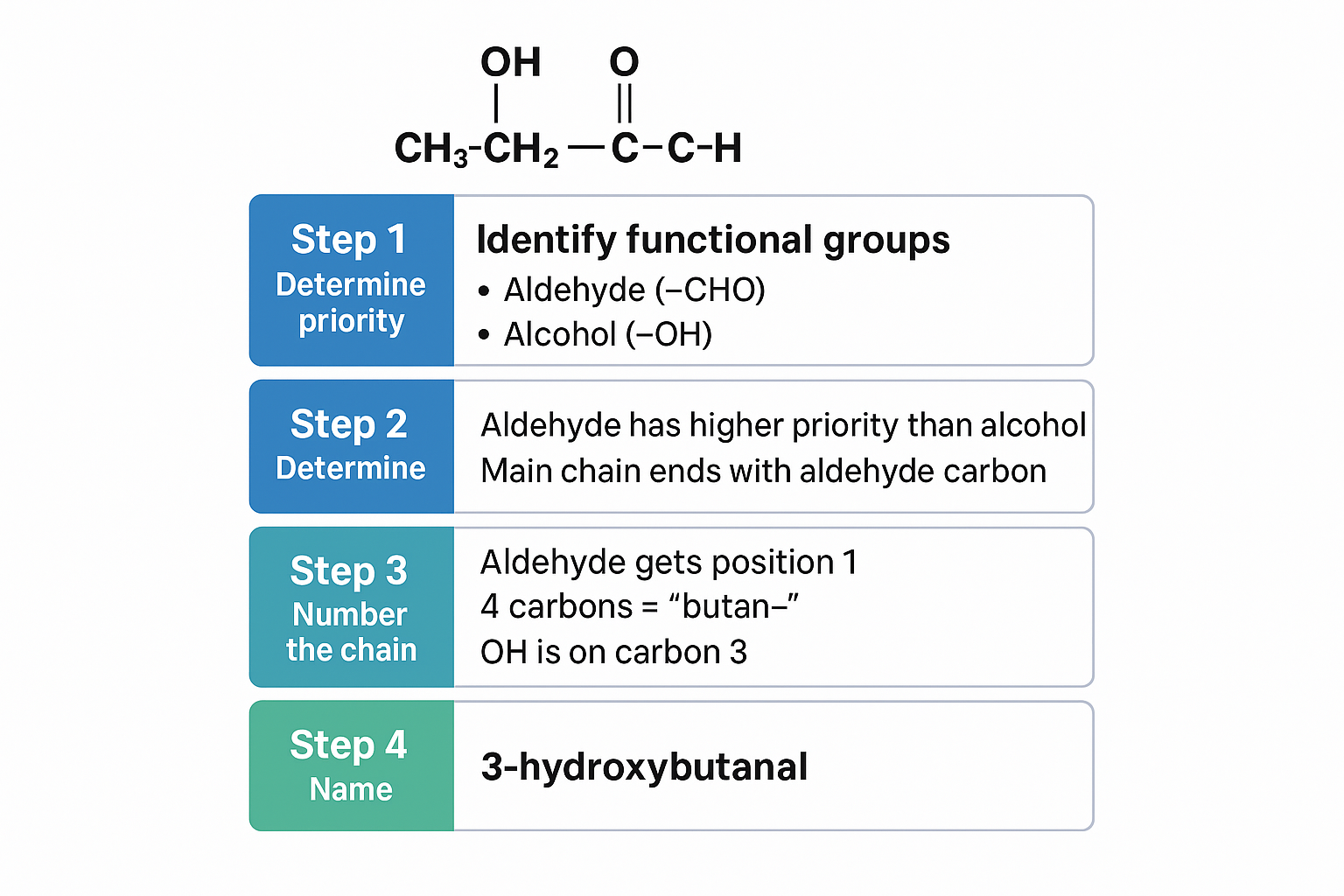
View original ASCII
OH O
│ ║
CH₃-CH₂-CH-C-H
Step 1: Identify functional groups
• Aldehyde (-CHO)
• Alcohol (-OH)
Step 2: Determine priority
• Aldehyde has higher priority than alcohol
• Main chain ends with aldehyde carbon
Step 3: Number the chain
• Aldehyde gets position 1
• 4 carbons = "butan-"
• OH is on carbon 3
Step 4: Name
• 3-hydroxybutanal
Answer: 3-hydroxybutanal
💡 Naming Strategy: Always identify the highest-priority functional group first—this determines the suffix and chain numbering direction.
Example 4: Perceptual Ability - Cube Counting 📦
Question Type: How many cubes have exactly 2 faces painted in this 3×3×3 stack where only the outer surface is painted?
Solution Process:
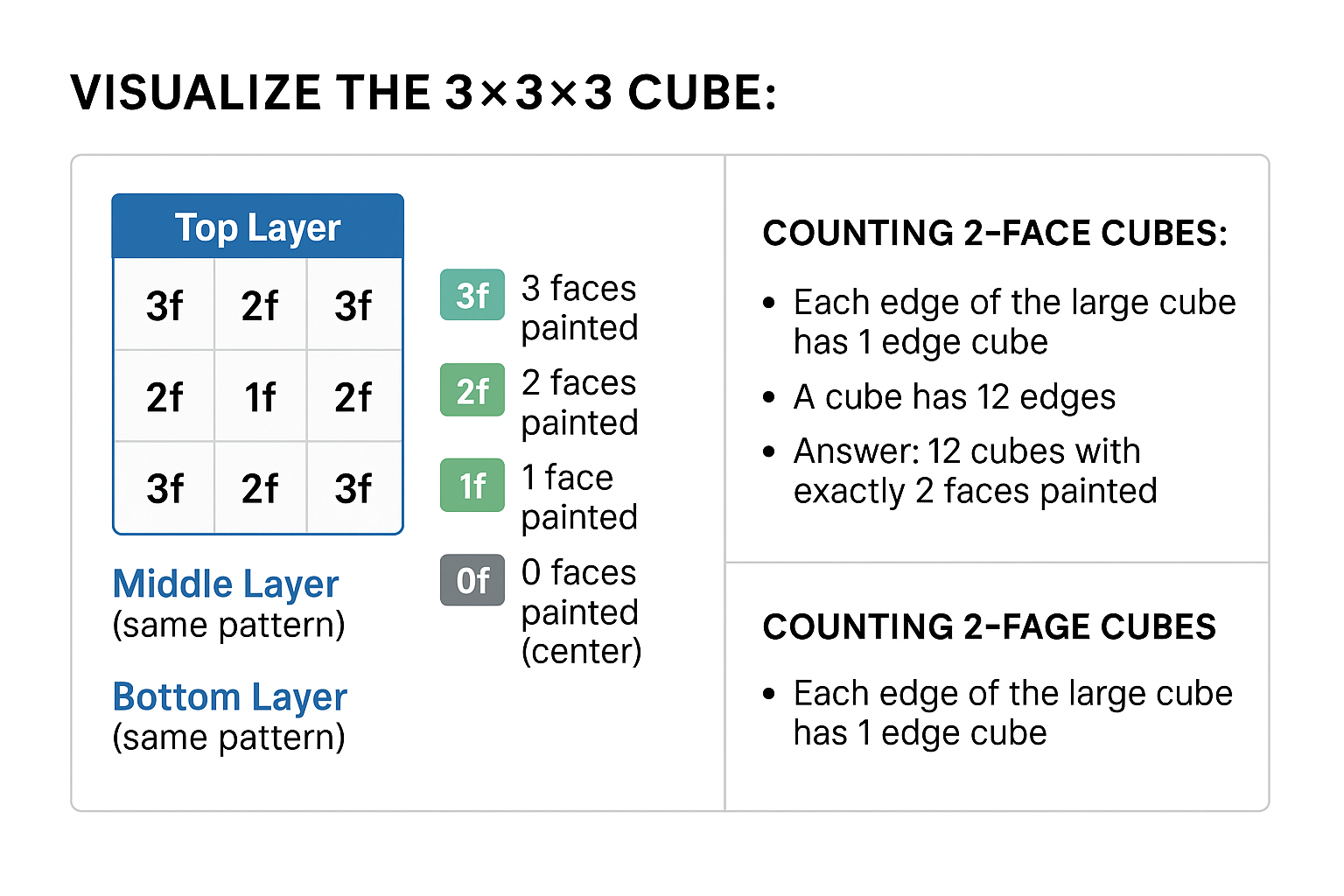
View original ASCII
VISUALIZE THE 3×3×3 CUBE:Top Layer ┌─────┬─────┬─────┐ │ 3f │ 2f │ 3f │ 3f = 3 faces painted (corners) ├─────┼─────┼─────┤ 2f = 2 faces painted (edges) │ 2f │ 1f │ 2f │ 1f = 1 face painted (faces) ├─────┼─────┼─────┤ 0f = 0 faces painted (center) │ 3f │ 2f │ 3f │ └─────┴─────┴─────┘Middle Layer (same pattern) Bottom Layer (same pattern)
COUNTING 2-FACE CUBES: • Each edge of the large cube has 1 edge cube • A cube has 12 edges • Answer: 12 cubes with exactly 2 faces painted
Answer: 12 cubes
💡 Universal Formula for n×n×n cube:
- Corner cubes (3 faces): Always 8
- Edge cubes (2 faces): 12(n-2)
- Face cubes (1 face): 6(n-2)²
- Internal cubes (0 faces): (n-2)³
Common Mistakes to Avoid ⚠️
Biology Mistakes
❌ Confusing mitosis and meiosis
- Mitosis = 2 daughter cells, diploid (2n), identical to parent
- Meiosis = 4 daughter cells, haploid (n), genetic variation
- 🧠 Mnemonic: "Meiosis makes megametes" (sex cells)
❌ Mixing up DNA vs RNA
- DNA: Deoxyribose sugar, thymine (T), double-stranded
- RNA: Ribose sugar, uracil (U), single-stranded
- Remember: RNA is "U"nique (has Uracil)
❌ Forgetting about negative feedback loops
- Most homeostatic mechanisms use negative feedback (not positive)
- Example: High blood glucose → insulin release → glucose drops → insulin stops
General Chemistry Mistakes
❌ pH calculation errors
- pH scale is logarithmic: pH 5 is 10× more acidic than pH 6
- Don't forget: pH + pOH = 14 (not pH + pOH = 7)
❌ Periodic trend confusion
- Atomic radius increases going DOWN and LEFT
- Electronegativity increases going UP and RIGHT
- They're opposite trends!
❌ Balancing equations prematurely
- Identify reaction type first (synthesis, decomposition, combustion, etc.)
- This helps predict products before balancing
Organic Chemistry Mistakes
❌ Ignoring stereochemistry
- Enantiomers have opposite biological activity
- DAT often asks about chiral centers in drug molecules
❌ Forgetting electron pushing
- Nucleophiles have electrons to donate (think: negative charges, lone pairs)
- Electrophiles accept electrons (think: positive charges, partial positive)
- Arrows go FROM nucleophile TO electrophile
❌ Misidentifying functional groups
- Aldehyde: -CHO (at the END of chain)
- Ketone: C=O (in the MIDDLE of chain)
- Don't confuse them!
Perceptual Ability Mistakes
❌ Not practicing enough
- PAT requires MUSCLE MEMORY, not just understanding
- Practice 30+ minutes daily for 2-3 weeks before test
❌ Second-guessing yourself
- Your first instinct on spatial problems is usually correct
- Only change answers if you find a clear error
❌ Spending too long on one problem
- Each PAT question should take 50-60 seconds
- Flag and move on if stuck
Key Takeaways 📚
🎯 Quick Reference Card: DAT Science Section
Time Management:
- Biology: 40 questions in ~35-40 min (highest priority!)
- Gen Chem: 30 questions in ~20-25 min
- Org Chem: 30 questions in ~20-25 min
- Reserve 5-10 min for review
High-Yield Biology Topics:
- Cell transport (active/passive)
- DNA replication & protein synthesis
- Mendelian genetics & inheritance
- Natural selection & evolution
- Anatomy (especially dental-relevant: muscles, nerves, blood vessels)
High-Yield General Chemistry:
- Stoichiometry & mole conversions
- pH/pOH calculations
- Periodic trends
- Thermodynamics (ΔH, ΔS, ΔG)
- Gas laws (PV=nRT)
High-Yield Organic Chemistry:
- Functional group identification
- IUPAC nomenclature
- Nucleophile vs electrophile
- Stereochemistry basics (R/S, E/Z)
- Common reactions (substitution, elimination, addition)
PAT Strategies:
- Angle ranking: Use 45° and 90° references
- Hole punching: Unfold in reverse order
- Cube counting: Use formulas for speed
- Pattern folding: Look for impossible configurations
- View recognition: Eliminate one wrong feature at a time
Test Day Tips:
- Use three-pass system (easy → medium → hard)
- Never leave questions blank (no penalty for guessing)
- Trust first instincts on spatial problems
- Check units on all calculations
- Remember: Breadth over depth—know fundamentals cold!
Further Study 📖
American Dental Association DAT Resources: https://www.ada.org/education-careers/dental-admission-test - Official test structure, practice materials, and registration
Khan Academy MCAT Prep (applicable to DAT): https://www.khanacademy.org/test-prep/mcat - Free comprehensive biology and chemistry review with practice questions
DAT Bootcamp: https://www.datbootcamp.com/ - Specialized DAT preparation with perceptual ability practice software and full-length practice tests
Ready to test your knowledge? The practice questions below will help reinforce these concepts and prepare you for DAT-style questions. Remember: focus on understanding principles, not memorizing facts. Good luck! 🦷✨